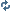|
Hi guys, I'm taking grade 12 chemistry right now and the teacher started with the introduction to Atomic Theory and its development. After thinking about all the stuff being taught, I am confused about certain concepts, and the teacher won't explain properly, hope someone can help me out.
The one big problem i have is that: we all know that the atom is made up of a nucleus and orbiting electrons. When an object is heated, it emits light. So according to my notes, it says that although electrons are orbiting around the nucleus and accelerating (change in direction because of its circular motion), and since its been scientifically proven that acceleration produce some type of light, the orbiting electrons would emit photons of electromagnetic radiation and lose energy, so theoretically they would crash into the nucleus.
So Bohr concluded that electrons have specific energy levels (electron shells), and if atoms absorb energy, the electrons will get excited and jump to a higher energy level, and when it returns to its "ground" position, the energy is released in the form of light.
The energy of a given energy level is determined by the following equation:
E = - R / n^2 ( where R is a constant of 2.18 x 10^-18 J, and "n" is the energy level/electron shell)
Here comes my problem, let's say I have a hydrogen atom (cuz its easy), its valence electrons are in n = 1 energy level. So if you plug it into the equation, you get a negative value. But the thing is, how can energy be negative?? or did i misinterpret the equation in some way?
ALso, it says that the energy of electron bound to the nucleus is lower than if the electron were at infinity, which imo makes sense. But next thing it says is that as n approaches infinity, Energy approaches ZERO. But how can that be? If n increases, doesn't energy also increase? so instead of the energy approaching zero, shouldn't it also approach infinity?
Hope you guys can help me out a little 
    
|
ok well, although I do expect someone on here to answer those questions correctly, you should really just go after class and ask your teacher these questions...
I can answer the infinity thing, though. So you plug in infinity for n. You get R over infinity. Infinity is a really big number. When you divide a small number by a big number, you get a VERY SMALL number. Since infinity is VERY BIG, you get a VERY VERY VERY VERY small number. So, it is treated as zero.
edit: ok my explanation might not seem clear.
What is 1 / 1,000,000,000? it's like .000000000001 or whatever. Now imagine instead of 1,000,000,000, you have infinity. So now you should get the idea
|
This is such a perfect question to ask in class though, doesn't make you look like an idiot, rather observant.
|
On September 05 2008 07:12 blabber wrote:
ok well, although I do expect someone on here to answer those questions correctly, you should really just go after class and ask your teacher these questions...
I can answer the infinity thing, though. So you plug in infinity for n. You get R over infinity. Infinity is a really big number. When you divide a small number by a big number, you get a VERY SMALL number. Since infinity is VERY BIG, you get a VERY VERY VERY VERY small number. So, it is treated as zero.
Yeah i asked my teacher twice, and she isn't (imo) a qualified chemistry teacher. All she does in class is read the notes provided by some other teacher probably and doesnt teach us anything. She got a bit impatient after being asked the same question twice without a solid response. :/
|
well if your teacher sucks, then you can always refer to your textbook... if you're still not getting it, that's when you should seek outside help
|
On September 05 2008 07:12 blabber wrote:
ok well, although I do expect someone on here to answer those questions correctly, you should really just go after class and ask your teacher these questions...
I can answer the infinity thing, though. So you plug in infinity for n. You get R over infinity. Infinity is a really big number. When you divide a small number by a big number, you get a VERY SMALL number. Since infinity is VERY BIG, you get a VERY VERY VERY VERY small number. So, it is treated as zero.
edit: ok my explanation might not seem clear.
What is 1 / 1,000,000,000? it's like .000000000001 or whatever. Now imagine instead of 1,000,000,000, you have infinity. So now you should get the idea
But what about the negative in front??
|
On September 05 2008 07:18 letsbefree wrote:Show nested quote +On September 05 2008 07:12 blabber wrote:
ok well, although I do expect someone on here to answer those questions correctly, you should really just go after class and ask your teacher these questions...
I can answer the infinity thing, though. So you plug in infinity for n. You get R over infinity. Infinity is a really big number. When you divide a small number by a big number, you get a VERY SMALL number. Since infinity is VERY BIG, you get a VERY VERY VERY VERY small number. So, it is treated as zero.
edit: ok my explanation might not seem clear.
What is 1 / 1,000,000,000? it's like .000000000001 or whatever. Now imagine instead of 1,000,000,000, you have infinity. So now you should get the idea But what about the negative in front??
That just means the number approaches zero from the negative side.
|
I use to know the answer to this, but its been a few years since I have done chemistry. If I had my notes or my textbooks I could help you but they are at my parent's house. Sorry. Have you tried a quick google search?
|
this is grade 12? more like grade 10.
first of all, electrons do not orbit the nucleus. that is the wrong way to visualize it. the electrons live in orbitals, and you can only determine the probability of finding the electron. you can never actually determine the definite point of a electron.
i kinda forgot, but im pretty sure the energy value is negative because thats saying the energy is released. think of it as change in energy. the change in energy is negative because energy is being released.
|
|
|
wow thanks a bunch guys, really appreciate it =')
|
Light is only emitted when the electrons change energy levels the amount of energy that is released is equal to your equation w/higher energy level plugged in minus (90% sure on this) the equation with the lower energy level.
dickless is right about everything.
You shouldn't trust me I can't get into chemistry at my retarded college even though I got a 5 on my AP test. /sarcasm /frustration
edit: for anyone who didn't know AP tests are test for college credit and the highest score is a 5.
|
The negative sign means the electrons are "bound" to the atom.
and as n increases, the electrons are farther away from the atom, so it makes sense that the pull of the nucleus decreases.
|
Basically, the Bohr model is wrong. Electrons do not orbit the nucleus like a planet does. The whole spinning into the nucleus thing was a problem that physicists had using the Bohr model because you have a positive charge in the center and electrons orbiting around it. That's all well and good, but the charges are opposite so there should be an attraction which slowly leads into the electron spiraling into the nucleus if what you detailed is true. This obviously does not happen.
As for "negative energy" that's a chemistry convention where if something is emitting energy, you put a negative sign in front of it. If it's absorbing energy it's positive. It's not actually "negative energy." Furthermore, the equation is not telling you the energy of the orbitals- it's giving you the energy of the transition between the orbitals. Big, big difference. It isn't that the energy approaches zero, the energy difference approaches zero. The emission spectra will show a series of lines. The lines start out fairly spaced out because the energy levels are pretty far apart to begin with, but then they start to converge in a mess of lines at some particular point and then end altogether.
The last line of this converging series of lines is the largest energy difference possible, which is n=infinity - n=1.
|

Bill307

Canada9103 Posts
On September 05 2008 07:06 letsbefree wrote:
The energy of a given energy level is determined by the following equation:
E = - R / n^2 ( where R is a constant of 2.18 x 10^-18 J, and "n" is the energy level/electron shell)
Here comes my problem, let's say I have a hydrogen atom (cuz its easy), its valence electrons are in n = 1 energy level. So if you plug it into the equation, you get a negative value. But the thing is, how can energy be negative?? or did i misinterpret the equation in some way?
The energy in question is simply potential energy. Don't fret over having negative potential energy. Another example of negative potential energy is gravitational potential energy, given by the equation:
E = - G m1 m2 / r
Mathematically, it still works. E.g. as an object falls from the sky (i.e. as r decreases), its gravitational potential energy decreases. Likewise, as an electron's energy level n decreases, its potential energy also decreases.
As for why these potential energies are negative... well, I'm not entirely sure. But at least in the case of gravitational potential energy, I do know there is a lot of physics-related math that depends on it being negative. So I assume in both cases, the negative sign is there because of the math involved.
|
On September 05 2008 07:59 Bill307 wrote:Show nested quote +On September 05 2008 07:06 letsbefree wrote:
The energy of a given energy level is determined by the following equation:
E = - R / n^2 ( where R is a constant of 2.18 x 10^-18 J, and "n" is the energy level/electron shell)
Here comes my problem, let's say I have a hydrogen atom (cuz its easy), its valence electrons are in n = 1 energy level. So if you plug it into the equation, you get a negative value. But the thing is, how can energy be negative?? or did i misinterpret the equation in some way? The energy in question is simply potential energy. Don't fret over having negative potential energy. Another example of negative potential energy is gravitational potential energy, given by the equation: E = - G m1 m2 / r Mathematically, it still works. E.g. as an object falls from the sky (i.e. as r decreases), its gravitational potential energy decreases. Likewise, as an electron's energy level n decreases, its potential energy also decreases. As for why these potential energies are negative... well, I'm not entirely sure. But at least in the case of gravitational potential energy, I do know there is a lot of physics-related math that depends on it being negative. So I assume in both cases, the negative sign is there because of the math involved.
We assign the value 0 to a certain level of magnitude arbitrarily. A value can be negative or positive depending on where you assigned the magnitude where it is 0. Just like height and potential energy. Sorry for my bad technical english 
OF course I could be wrong, but I think that's it.
|
Or it's because the chemists claim dominion over the quantum world... mwhaha.
|

Bill307

Canada9103 Posts
On September 05 2008 07:45 zer0das wrote:
As for "negative energy" that's a chemistry convention where if something is emitting energy, you put a negative sign in front of it. If it's absorbing energy it's positive. It's not actually "negative energy." Furthermore, the equation is not telling you the energy of the orbitals- it's giving you the energy of the transition between the orbitals. Big, big difference. It isn't that the energy approaches zero, the energy difference approaches zero. The emission spectra will show a series of lines. The lines start out fairly spaced out because the energy levels are pretty far apart to begin with, but then they start to converge in a mess of lines at some particular point and then end altogether.
This (specifically the part in bold) is incorrect: it is in fact a simplified form of the equation for the energy of an electron in an orbital around an atom. See here:
http://en.wikipedia.org/wiki/Energy_level#Orbital_state_energy_level
If you keep Z constant, then you can simplify the equation so that there's just one constant "R".
|

Bill307

Canada9103 Posts
On September 05 2008 08:07 kemoryan wrote:Show nested quote +On September 05 2008 07:59 Bill307 wrote:On September 05 2008 07:06 letsbefree wrote:
The energy of a given energy level is determined by the following equation:
E = - R / n^2 ( where R is a constant of 2.18 x 10^-18 J, and "n" is the energy level/electron shell)
Here comes my problem, let's say I have a hydrogen atom (cuz its easy), its valence electrons are in n = 1 energy level. So if you plug it into the equation, you get a negative value. But the thing is, how can energy be negative?? or did i misinterpret the equation in some way? The energy in question is simply potential energy. Don't fret over having negative potential energy. Another example of negative potential energy is gravitational potential energy, given by the equation: E = - G m1 m2 / r Mathematically, it still works. E.g. as an object falls from the sky (i.e. as r decreases), its gravitational potential energy decreases. Likewise, as an electron's energy level n decreases, its potential energy also decreases. As for why these potential energies are negative... well, I'm not entirely sure. But at least in the case of gravitational potential energy, I do know there is a lot of physics-related math that depends on it being negative. So I assume in both cases, the negative sign is there because of the math involved. We assign the value 0 to a certain level of magnitude arbitrarily. A value can be negative or positive depending on where you assigned the magnitude where it is 0. Just like height and potential energy. Sorry for my bad technical english  OF course I could be wrong, but I think that's it.
I think you're wrong. If you look at the page I linked above, since gravity is a conservative force, point #3 basically says the gravitational force must be the negative derivative of the gravitational potential energy, with respect to r. Therefore, the equation for the gravitational potential energy must have that form: we cannot arbitrarily move the 0 point anywhere.
|
On September 05 2008 08:07 Bill307 wrote:Show nested quote +On September 05 2008 07:45 zer0das wrote:
As for "negative energy" that's a chemistry convention where if something is emitting energy, you put a negative sign in front of it. If it's absorbing energy it's positive. It's not actually "negative energy." Furthermore, the equation is not telling you the energy of the orbitals- it's giving you the energy of the transition between the orbitals. Big, big difference. It isn't that the energy approaches zero, the energy difference approaches zero. The emission spectra will show a series of lines. The lines start out fairly spaced out because the energy levels are pretty far apart to begin with, but then they start to converge in a mess of lines at some particular point and then end altogether. This (specifically the part in bold) is incorrect: it is in fact a simplified form of the equation for the energy of an electron in an orbital around an atom. See here: http://en.wikipedia.org/wiki/Energy_level#Orbital_state_energy_levelIf you keep Z constant, then you can simplify the equation so that there's just one constant "R".
So umm...can you help explain my questions?? I'm really confused nowwww
|
|
|

Bill307

Canada9103 Posts
Okay, let me try to be more understandable.
On September 05 2008 07:06 letsbefree wrote:
The energy of a given energy level is determined by the following equation:
E = - R / n^2 ( where R is a constant of 2.18 x 10^-18 J, and "n" is the energy level/electron shell)
Here comes my problem, let's say I have a hydrogen atom (cuz its easy), its valence electrons are in n = 1 energy level. So if you plug it into the equation, you get a negative value. But the thing is, how can energy be negative?? or did i misinterpret the equation in some way?
ALso, it says that the energy of electron bound to the nucleus is lower than if the electron were at infinity, which imo makes sense. But next thing it says is that as n approaches infinity, Energy approaches ZERO. But how can that be? If n increases, doesn't energy also increase? so instead of the energy approaching zero, shouldn't it also approach infinity?
Firstly, I'm 90% sure the energy here is potential energy. Potential energy can be negative because it's not really a physical quantity. In my experience, it's probably negative because the really complex math behind it all requires the potential energy to be negative.
Aside from the fact that it's potential energy, your interpretation is correct.
If n increases, the potential energy increases towards 0. (Remember: it's negative. Example: -1 is less than 0, so you can increase from -1 to 0.)
|
the negative means energy is released (exothermic)
i've never used this equation though, but i remember using bohr's equation for electrons moving to different energy levels in a hydrogen atom
E = -2.178x10^-19 ( Z^2/n^2)
where n is the energy level and z is the nuclear charge (always 1, this only works for hydrogen because bohr's model is fundamentally wrong)
if n is infinity, then it would be incredibly far away from the nucleus and there would be no real attraction between them, making the energy 0.
|

Bill307

Canada9103 Posts
Why am I the only person who doesn't try to explain away why the energy is negative? In this situation, it has nothing to do with changes in energy or energy being absorbed / released: the potential energy really is negative.
I don't know what you guys are gonna do when you encounter gravitational potential energy, which is negative as well. ~_~
|
On September 05 2008 08:35 Bill307 wrote:
Why am I the only person who doesn't try to explain away why the energy is negative? In this situation, it has nothing to do with changes in energy or energy being absorbed / released: the potential energy really is negative.
I don't know what you guys are gonna do when you encounter gravitational potential energy, which is negative as well. ~_~
err actually i was thinking a few steps ahead of the problem where you would get the energy levels for them and find the change in them, and that would come out negative (because it's exothermic)
the negative is because... you were right it actually is negative. smaller = farther away from nucleus... so yeah. my bad.
|
On September 05 2008 08:35 Bill307 wrote:
Why am I the only person who doesn't try to explain away why the energy is negative? In this situation, it has nothing to do with changes in energy or energy being absorbed / released: the potential energy really is negative.
I don't know what you guys are gonna do when you encounter gravitational potential energy, which is negative as well. ~_~
Take more chemistry, we don't really give a rip about your conventions and that's what ours say.
Anywho... yes you can calculate the energy of a level, but the one that is spit out when n=infinity makes zero (harhar) sense. And here's why:
Because the electron has been ejected from the atom. It is the lowest "unbound state", so it quite simply isn't there. Hence it makes a lot more sense to think of it as a difference of energies in this case (and in the general case so you can interpret this situation).
And here's what my PChem book says on the positive and negatives: "All the energies are negative. They refer to the bound states of the atom, in which the energy of the atom is lower than the infinitely separated, stationary electron and nucleus (note, this is an assumption, they aren't actually stationary) which correspond to the zero energy. There are also solutions of the Schroedinger equation with positive energies. These solution correspond to the unbound states of the electron, the states to which an electron is raised when it is ejected from the atom by a high-energy collision or photon)."
I also have notes from that class stating that n=infinity is the first unbound state. BUt logically this must be so since zero is defined as occurring when the nucleus and electron are infinitely far apart.
But I can guarantee almost every chemist thinks of it in terms of energy being emitted if the sign is negative, because if it's an emission spectra, it is. And quite frankly spectroscopy is the useful application of this stuff.
|

United States24753 Posts
On September 05 2008 08:07 zer0das wrote:
Or it's because the chemists claim dominion over the quantum world... mwhaha.
Find me a chemist who can calculate the Bohr radius in terms of fundamental constants (without cheating) and I'll find you a physics teacher who is really good at starcraft.
|
On September 05 2008 07:06 letsbefree wrote:Here comes my problem, let's say I have a hydrogen atom (cuz its easy), its valence electrons are in n = 1 energy level. So if you plug it into the equation, you get a negative value. But the thing is, how can energy be negative?? or did i misinterpret the equation in some way? ALso, it says that the energy of electron bound to the nucleus is lower than if the electron were at infinity, which imo makes sense. But next thing it says is that as n approaches infinity, Energy approaches ZERO. But how can that be? If n increases, doesn't energy also increase? so instead of the energy approaching zero, shouldn't it also approach infinity? Hope you guys can help me out a little 
Anyways, to concisely answer your question: you're confusing the energy of a particular level and the energy difference between levels. If you read the definition I posted above, if the electron and nucleus are infinitely far apart, the energy is defined as zero. At n=infinity the electron and the nucleus are infinitely far apart, so the energy of that level is 0. The energy to move an electron from n=1 to n=infinity is not zero.
The energy to move an electron from n=1 to n=infinity is still finite, because it is a difference in energy levels. In this case the difference between whatever the energy of n=1 is and 0. This makes sense, since it's basically saying you need to put in as much energy to eject an electron as there is holding it there.
Also at n=infinity, the electron has been ejected from the atom. That's why in the spectra the lines converge to a certain point and at the last line n=infinity (although it actually isn't, but that's the best you can do). After it has been ejected, the electron's emission spectrum can no longer be measured because it is simply gone (it needs to relax to a lower energy level to give off energy, which it can no longer do... at least until it smacks into an atom that is deficient in electrons).
Hope that helps. :d
|
On September 05 2008 09:10 micronesia wrote:Show nested quote +On September 05 2008 08:07 zer0das wrote:
Or it's because the chemists claim dominion over the quantum world... mwhaha. Find me a chemist who can calculate the Bohr radius in terms of fundamental constants (without cheating) and I'll find you a physics teacher who is really good at starcraft.
Funny.. my PChem teacher made us do that. -_-
We did get to see the constants though, and we were given a spectra with some data. But yeah. ;p
|
As for why these potential energies are negative... well, I'm not entirely sure.
I am.
Its pretty obvious. When an electron starts orbiting a nucleus, the relationship is at a lower energy level than previously. Since you're in a potential energy 'pit' so to speak, the current energy of the system is negative with respect to an electron and an atom at infinity distance apart. Basically you're measuring the amount of energy RELEASED by the system in forming the 'bond' if you will, and thus the system has less energy than when it started after the bonding is complete and the excess bond energy has been radiated away via light, heat, kinetics, or whatever other path it uses for relaxation.
Nitrogen seems to be correct but i only read his first post.
Find me a chemist who can calculate the Bohr radius in terms of fundamental constants (without cheating) and I'll find you a physics teacher who is really good at starcraft.
I can do that?
Once you know to use the gas law (which IS cheating) + electrostatic attraction + angular momentum, its pretty much done.
|

United States24753 Posts
I won't accept anything short of solving the Schrodinger Equation for the Hydrogen Atom.
|
On September 05 2008 08:10 Bill307 wrote:Show nested quote +On September 05 2008 08:07 kemoryan wrote:On September 05 2008 07:59 Bill307 wrote:On September 05 2008 07:06 letsbefree wrote:
The energy of a given energy level is determined by the following equation:
E = - R / n^2 ( where R is a constant of 2.18 x 10^-18 J, and "n" is the energy level/electron shell)
Here comes my problem, let's say I have a hydrogen atom (cuz its easy), its valence electrons are in n = 1 energy level. So if you plug it into the equation, you get a negative value. But the thing is, how can energy be negative?? or did i misinterpret the equation in some way? The energy in question is simply potential energy. Don't fret over having negative potential energy. Another example of negative potential energy is gravitational potential energy, given by the equation: E = - G m1 m2 / r Mathematically, it still works. E.g. as an object falls from the sky (i.e. as r decreases), its gravitational potential energy decreases. Likewise, as an electron's energy level n decreases, its potential energy also decreases. As for why these potential energies are negative... well, I'm not entirely sure. But at least in the case of gravitational potential energy, I do know there is a lot of physics-related math that depends on it being negative. So I assume in both cases, the negative sign is there because of the math involved. We assign the value 0 to a certain level of magnitude arbitrarily. A value can be negative or positive depending on where you assigned the magnitude where it is 0. Just like height and potential energy. Sorry for my bad technical english  OF course I could be wrong, but I think that's it. I think you're wrong. If you look at the page I linked above, since gravity is a conservative force, point #3 basically says the gravitational force must be the negative derivative of the gravitational potential energy, with respect to r. Therefore, the equation for the gravitational potential energy must have that form: we cannot arbitrarily move the 0 point anywhere.
Actually, he's right; what matters is not the overall number of any energy level, just the difference between levels. The page you mention is also valid. But, if you take a look at the equation you mention, you can see that adding a constant number to the potential energy (represented by phi) won't change the force at all, and so the physics will still be preserved.
This is analogous to saying that the integral of a particular function can only be determined up to a constant (i.e. the integral of 2x is x^2 + c, where c can be anything). This is something that particularly snooty physicists might call gauge invariance.
And the reason the energies are negative here, as some people have already mentioned, is that we choose to define the energy of a free electron as zero, just for convenience. Therefore, because it takes energy to remove an electron from any energy level, those energy levels have to be negative (think of the potential energy as elevation/altitude... since we're "below sea level" we need to add energy to the electron to get back up to sea level).
|
On September 05 2008 07:06 letsbefree wrote:
The one big problem i have is that: we all know that the atom is made up of a nucleus and orbiting electrons. When an object is heated, it emits light. So according to my notes, it says that although electrons are orbiting around the nucleus and accelerating (change in direction because of its circular motion), and since its been scientifically proven that acceleration produce some type of light, the orbiting electrons would emit photons of electromagnetic radiation and lose energy, so theoretically they would crash into the nucleus.
This is wrong on so many levels...
Firstly objects producing electromagnetic waves due to temperature levels have nothing to do with electron orbits but instead the atoms movements. This is very important since heat radiation do not follow the same rules at all but instead just radiates electromagnetic waves of a distribution curve were the peak is determined by the temperature.
The waves you are talking about are instead produced when you flow electrons through for example gases, this causes the electrons already bound by the gas to get knocked away by other electrons, and it is when these empty electron orbits gets filled the electrons are accelerated and an exact wavelength of light is emitted according to the difference in potential energy between the two points. (Potential energy is 0 furthest away while a low level orbit is -a, then a jump to the low level orbit gives 0-(-a)=a amount of energy which discharges the "photon" with an energy amount of exactly a)
You can see the difference between these two light sources with everything which can create a spectrum. If you look at a neon light through a spectrum you will find that it only emits light in a single frequency, while light bulbs, which operate by heating the thread, emits of every visible frequency and thus creates the whole visible spectrum. This is the reason light bulbs are so inefficient while lamps that use electron jumps are much nicer on your electricity bills since the amount of heat needed creates a lot of waste while electron jumps can operate at low temperatures.
Also you use the same technique to see which elements the stars are made of, since its only black body radiation which gives all of the light spectrum, every other source emits everything but the frequencies which corresponds to the electron cloud orbits which gives holes in the spectrum created. This gives us a very exact method of finding out what everything's surface is made of and together with the Doppler effect also the relative speed to every visible spot in the universe.
|
On September 05 2008 09:19 zer0das wrote:Show nested quote +On September 05 2008 07:06 letsbefree wrote:Here comes my problem, let's say I have a hydrogen atom (cuz its easy), its valence electrons are in n = 1 energy level. So if you plug it into the equation, you get a negative value. But the thing is, how can energy be negative?? or did i misinterpret the equation in some way? ALso, it says that the energy of electron bound to the nucleus is lower than if the electron were at infinity, which imo makes sense. But next thing it says is that as n approaches infinity, Energy approaches ZERO. But how can that be? If n increases, doesn't energy also increase? so instead of the energy approaching zero, shouldn't it also approach infinity? Hope you guys can help me out a little  Anyways, to concisely answer your question: you're confusing the energy of a particular level and the energy difference between levels. If you read the definition I posted above, if the electron and nucleus are infinitely far apart, the energy is defined as zero. At n=infinity the electron and the nucleus are infinitely far apart, so the energy of that level is 0. The energy to move an electron from n=1 to n=infinity is not zero. The energy to move an electron from n=1 to n=infinity is still finite, because it is a difference in energy levels. In this case the difference between whatever the energy of n=1 is and 0. This makes sense, since it's basically saying you need to put in as much energy to eject an electron as there is holding it there. Also at n=infinity, the electron has been ejected from the atom. That's why in the spectra the lines converge to a certain point and at the last line n=infinity (although it actually isn't, but that's the best you can do). After it has been ejected, the electron's emission spectrum can no longer be measured because it is simply gone (it needs to relax to a lower energy level to give off energy, which it can no longer do... at least until it smacks into an atom that is deficient in electrons). Hope that helps. :d
This is correct. Here's a a picture to help you (OP) visualize. 
Also, R is supposed to be negative.. but yeah. You get the point.
Also, last point is if N=1 for the electron. If it's in a different shell, the (ionization) energy is less for the electron to escape.
![[image loading]](http://img513.imageshack.us/img513/5026/electronsandemtf8.png)
P.S. Dotted lines mean nothing at all. I just put them there to show the exponential relationship of the energy of N shells since they're based on -R/N^2
|
|
|
|
|
|








![[image loading]](http://img513.imageshack.us/img513/5026/electronsandemtf8.png)