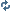|
It was only two years ago that IBM showed us an image of a complete molecule, atomic bonds and all, but today's news does that one infinitesimally-sized breakthrough better. Ladies and gents, behold the first image of an electron's path.
![[image loading]](http://i.imgur.com/5vixq.jpg)
SOURCE: http://gizmodo.com/5835164/fascinatingly-small-images-give-first ever-glimpse-of-an-electrons-orbit
Full Article: http://www.nature.com/nchem/journal/v3/n4/full/nchem.1008.html#/access
Explanation: On August 30 2011 06:49 Nycaloth wrote:ok.... i see there is some explaining to do here. just as a bit of a reference, i work as a PhD student for one of the people who helped obtain the results referenced in this http://gizmodo.com/5346964/ibm-takes-first-3d-image-of-atomic-bonds] article, which is also linked in the gizmodo article in the first post and work in the field of molecular microscopy. There are some misconceptions and presented so far and id like to rectify that a bit and give a very general explanation on what we see and what it means. The pictures we see are taken by very advanced microcopes, the techniques used are scanning tunneling mocroscopy (STM) and atomic force microscopy (AFM). though called that, they are not microscopes in the "traditional" sense and do not use light and lenses to peek into the very small. instead, both work by scanning a surface with a very narrow metal tip and recording certain surface properties at different points. these measurements can be combined into pictures of the surface, single measurements being the pixels combining the picture. Through the use of piezo crystals and sofisticated dampening of the tip-sample system as a whole, the motion of the tip can be controlled very precisely, on scales smaller the the diameter of an atom. understanding of both techniques requires a basic familiarity with quantum mechanics, but the wikipedia articles on the subject should be enough to explain how exactly they work. Since we are doing quantum mechanics (QM), one has to be exceedingly careful when using terms such as "position", "velocity" or "path", since they cannot usually be defined in a meaningful way any more. i cant read the full article referenced in the OP at the moment since im not at the university and dont have access to it, but what we see in the pictures are molecular orbitals. what QM can only predict possibilities and other statistical quantities. orbitals are such a quantity, they represent the possibility to find an electron within a certain volume of space. this quantity can be computed numerically for complex systems such as molecules and these days can be measured by STM. due to electronic properties, namely the pauli exclusion principle, only two electrons can fit into one of these orbitals at a time. they are filled up until all the electrons in the molecule are used up. Thus, wer get the highest occupied molecular orbital, the HOMO, and the lowest unoccupied moelcular orbital, or LUMO. these two are usually involved when the molecule exchanges charge with its surroundings, eg during the formation of chemical bonds, which makes them interesting for study. what can we learn from this? well, by studying the electronic properties of molecules, we can learn something about their behaviour! and since the STM and AFM also allow us to manipulate single atoms and molecules, we can start building things on this scale. our hope is that by understanding molecular interaction on this small scale, we will be able to build circuitry, memory or other useful things from molecular building blocks. a few advances have already been made in this area, as researchers have found molecules that can act as switches or rectifiers or have other potentially useful properties. ill be happy to try and answer any questions on the topic!
This is simply amazing to be able to prove all those electron models, its crazy how fast camera's are advancing. Just to clarify the electrons are densest in the white parts and the nuclei is somewhere in the middle of all that.
|
That's pretty incredible. Do you have a source?
|
So...it would be swell to have some sources for this amazing new information.
|
Could someone tell me what I'm looking at exactly. What are the white parts / what are the black parts / what is the relation between the pictures?
|
Untill I see a source I believe those are my dentist xrays.
After source I will be very interested indeed.
EDIT: This is awesome. I wonder how small they can get the exposure in 10 years.
|
Honey I shrunk the camera.
|
|
|
Some explanation on what this is exactly ? I can't quite make sense of these photos with my very limited knowledge on the matter
|
Very nice, but there are better sources than Gizmodo for that stuff =D
|
This is HUGE in terms of quantum physics. Imagine the changes in chemistry and intramolecular physics with the advancements of tracking electron's paths. The next few months with this are going to be quite interesting.
|
For those of you currently in university, you can read the original paper if you go through your school's network or provided VPN.
This amazing, thanks for sharing.
|
|
|
On August 30 2011 03:09 Djzapz wrote:
Very nice, but there are better sources than Gizmodo for that stuff =D
The full article underneath goes into more about it
|
On August 30 2011 03:04 DisneylandSC wrote:
Could someone tell me what I'm looking at exactly. What are the white parts / what are the black parts / what is the relation between the pictures?
The white parts are electron clouds I guess you could call it and the black part is just background or something I suppose. It's pretty much proof of that the theory of how the electrons looklike in orbit looks like, which the pictures below show how they look like in theory.
I fail at explaining :<
On August 30 2011 03:11 shindigs wrote:
For those of you currently in university, you can read the original paper if you go through your school's network or provided VPN.
This amazing, thanks for sharing.
Oeh, thanks for the advice. Have to see if I can find it ^^
|
United Arab Emirates116 Posts
This is cosmically epic!
Grats to human innovation!
If only we could make that time machine work! :D
|
not really understand the images, if its true its amazing though electrons don't follow an orbit, ithey are pretty much at random places inside an orbital
|
Comments in the article point out, they have not taken any image of an electron, the picture you're seeing is the deflection from the entire orbit of the electron. This is a significant difference in size by many orders of magnitude.
|
Great to see people still pursuing knowledge for knowledge's sake.
|
On August 30 2011 03:15 ilj.psa wrote:
not really understand the images, if its true its amazing though electrons don't follow an orbit, ithey are pretty much at random places inside an orbital
That's been known for about 100 years now...
|
On August 30 2011 03:18 0mar wrote:Show nested quote +On August 30 2011 03:15 ilj.psa wrote:
not really understand the images, if its true its amazing though electrons don't follow an orbit, ithey are pretty much at random places inside an orbital That's been known for about 100 years now...
Yes, I was just pointing that out since the OP title is misleading
|
I really love the "carbon dioxide atom" that is mentioned multiple times in the link labeled "Explanation".
|
On August 30 2011 03:09 Nqsty wrote:
Some explanation on what this is exactly ? I can't quite make sense of these photos with my very limited knowledge on the matter
The white portions are "electron clouds", but a more telling name would be electron probability clouds. To use the word "orbit" is sometimes misleading, because electrons don't orbit around atoms or molecules like planets orbit around the sun. Locating the position of an electron is best if you try to estimate the probability of where it will be at a particular time. These are what those clouds represent. Why this is amazing is the images they generated match the mathematical theory and models that we used to attempt to visualize these "orbitals".
HOMO = Highest occupied molecular orbitals (the highest energy level where there are electrons)
LUMO = Lowest unoccupied molecular orbital (the lowest energy where there are no electrons)
--------- <----LUMO
----e---- <---- HOMO if e were to represent electrons
-----e---
-----e----
|
The white part in the picture you are looking at is the region called the orbital....It is basically where the electron spends most of its time.....An electron is CONSTANTLY moving at incredible speeds.....they move around the nucleus of the atom....the black part is where the electron is NOT and the white part is where the electron is rotating around said nucleus.....this is freakin incredible!!! Back in organic chemistry my professor used to say things like "until we can see an actual electron path, we can only theorize and guess, and apply the model that fits the best".....awesome stuff HOORAY! for science!~
|
I assume the bottom picture is theory and the top is empirical? If so, that is really cool. Great to see many years of theory finally get validated with empirical evidence.
Until now we've essentially been taking it with a pinch of faith, because the math worked.
|
On August 30 2011 03:28 Bibdy wrote:
I assume the bottom picture is theory and the top is empirical? If so, that is really cool. Great to see many years of theory finally get validated with empirical evidence.
Until now we've essentially been taking it with a pinch of faith, because the math worked.
correct. the top is the actual images and the bottom is the predictions of the mathematical model. It's incredible how accurately they match.
On August 30 2011 03:38 GreEny K wrote:
I guess it's a huge leap... Still looks like fuzz balls to me though. Guess you need an appreciation for for science and all that.
Yeah, if you don't know anything about the subject, this will likely mean very little to you.
|
I guess it's a huge leap... Still looks like fuzz balls to me though. Guess you need an appreciation for for science and all that.
|
On August 30 2011 03:38 GreEny K wrote:
I guess it's a huge leap... Still looks like fuzz balls to me though. Guess you need an appreciation for for science and all that.
Which you don't have…? Or what are you trying to say? o_o
|
So incredible. It's amazing to see that and realize thats not just a mathematical model, thats a fucking picture. Nerd chills
|
Nice, really looking forward to the next step.
|
The incredible thing here is that the model matches perfectly with the experimental data, beautiful!
|
This is truly impressive, especially the "microscope" technology involved. Let me quote that part of the article:
An atomic force microscope (AFM) isn't anything like an optical microscope that you simply look through to make small things appear bigger. Instead, it's more like a very very very very very small bit of charcoal that you can rub on tracing paper placed over a surface to view carved patterns that you wouldn't otherwise be able to see. The tip of an AFM (also called a probe) is so small that you need an electron microscope to even see it: To operate, the tip of the AFM moves across a surface, and when it encounters an atom or a molecule, the tip bumps up a little bit as it passes over. This jiggles a laser beam, which records precisely how much the tip was deflected. By making a bunch of passes, the AFM can gradually build up a sort of topographic map of a surface. It's also possible to place a single atom on the very tip of the AFM's probe, and by watching how that atom interacts with the atoms that it passes over, you can tell what's underneath.
Just imagine the level of precision neccessary to develop, manufacture, operate and record all of that. And think about the possibilites we could have if such machines couldn't just be used to scan, but instead to modify or create things on that scale.
|
Did they really need to label that picture HOMO? Surely a better acronym could have been found~
|
On August 30 2011 03:49 insaneMicro wrote:
Did they really need to label that picture HOMO? Surely a better acronym could have been found~
Why? Because some 12year old kids might giggle at it?
|
On August 30 2011 03:46 Shockk wrote:This is truly impressive, especially the "microscope" technology involved. Let me quote that part of the article: Show nested quote +An atomic force microscope (AFM) isn't anything like an optical microscope that you simply look through to make small things appear bigger. Instead, it's more like a very very very very very small bit of charcoal that you can rub on tracing paper placed over a surface to view carved patterns that you wouldn't otherwise be able to see. The tip of an AFM (also called a probe) is so small that you need an electron microscope to even see it: To operate, the tip of the AFM moves across a surface, and when it encounters an atom or a molecule, the tip bumps up a little bit as it passes over. This jiggles a laser beam, which records precisely how much the tip was deflected. By making a bunch of passes, the AFM can gradually build up a sort of topographic map of a surface. It's also possible to place a single atom on the very tip of the AFM's probe, and by watching how that atom interacts with the atoms that it passes over, you can tell what's underneath. Just imagine the level of precision neccessary to develop, manufacture, operate and record all of that. And think about the possibilites we could have if such machines couldn't just be used to scan, but instead to modify or create things on that scale.
This is what always excites me about atomic and molecular studies.
|
On August 30 2011 03:46 Shockk wrote:This is truly impressive, especially the "microscope" technology involved. Let me quote that part of the article: Show nested quote +An atomic force microscope (AFM) isn't anything like an optical microscope that you simply look through to make small things appear bigger. Instead, it's more like a very very very very very small bit of charcoal that you can rub on tracing paper placed over a surface to view carved patterns that you wouldn't otherwise be able to see. The tip of an AFM (also called a probe) is so small that you need an electron microscope to even see it: To operate, the tip of the AFM moves across a surface, and when it encounters an atom or a molecule, the tip bumps up a little bit as it passes over. This jiggles a laser beam, which records precisely how much the tip was deflected. By making a bunch of passes, the AFM can gradually build up a sort of topographic map of a surface. It's also possible to place a single atom on the very tip of the AFM's probe, and by watching how that atom interacts with the atoms that it passes over, you can tell what's underneath. Just imagine the level of precision neccessary to develop, manufacture, operate and record all of that. And think about the possibilites we could have if such machines couldn't just be used to scan, but instead to modify or create things on that scale.
WOO nano machines!!! GOGO BORG!!!
|
On August 30 2011 03:46 Shockk wrote:This is truly impressive, especially the "microscope" technology involved. Let me quote that part of the article: Show nested quote +An atomic force microscope (AFM) isn't anything like an optical microscope that you simply look through to make small things appear bigger. Instead, it's more like a very very very very very small bit of charcoal that you can rub on tracing paper placed over a surface to view carved patterns that you wouldn't otherwise be able to see. The tip of an AFM (also called a probe) is so small that you need an electron microscope to even see it: To operate, the tip of the AFM moves across a surface, and when it encounters an atom or a molecule, the tip bumps up a little bit as it passes over. This jiggles a laser beam, which records precisely how much the tip was deflected. By making a bunch of passes, the AFM can gradually build up a sort of topographic map of a surface. It's also possible to place a single atom on the very tip of the AFM's probe, and by watching how that atom interacts with the atoms that it passes over, you can tell what's underneath. Just imagine the level of precision neccessary to develop, manufacture, operate and record all of that. And think about the possibilites we could have if such machines couldn't just be used to scan, but instead to modify or create things on that scale.
AFM microscopes with near atomic resolutions has been around for a couple of decades now, we spent a good couple of weeks at first year of my physics bachelor using simple AFM microscopes to look at gold plates, or find carbonnanotubes on gold substrates.
AFM microscopes work both ways and are frequently used to manipulate atoms in various ways.The reason that it's not really used much for anything practical is that it's extremely tedious work. Like this for instance (manipulation of a tin surface and replacing with silicon atoms):
![[image loading]](http://metamodern.com/b/wp-content/uploads/2009/03/atom_interchange_anim.gif)
A couple of the first results from google about the subject:
http://www.nanowerk.com/spotlight/spotid=1168.php
http://metamodern.com/2009/03/14/afm-atom-manipulation-a-surprising-technique/
The reason that it's not really used much for anything practical is that it's extremely tedious work.
|
|
|
Wow, incredible. Seeing this really made my day.
|
The middle of image C has flickering points which are also very fascinating.
|
IF i remember correctly the equations that give rise to the shape of these atomic orbitals are found by separating the variables in Schrödinger's equation. This separation is made possible assuming that time and space dimensions are perpendicular to each other. Now that the atomic orbitals are justified... Food for thought!
|
The best part is the fig. A called HOMO
|
On August 30 2011 03:06 travis wrote:
Honey I shrunk the camera.
wow, i lol'd xD
pretty cool pics tho!
|
wow - epic pics... this really blows my mind away
|
On August 30 2011 03:41 enzym wrote:Show nested quote +On August 30 2011 03:38 GreEny K wrote:
I guess it's a huge leap... Still looks like fuzz balls to me though. Guess you need an appreciation for for science and all that. Which you don't have…? Or what are you trying to say? o_o
I think he was probably trying to say that you have to understand the significance of the discovery in order to appreciate the magnitude of the breakthrough.
But I am not science-savvy at all, so I am less blown-away by the images, and I think he feels the same way.
|
hm maybe my 12th class chemistry was not all just bollocks after all ...
|
I just had orbital theories in my chemistry class. Friday I have to write the semester exam about it (with other things like Schrödinger, Heisenberg, Planck stuff).
Really nice to see it with "real" pictures and not just modells
|
hmm this makes me feel stupid, time to research this, to feel better.
|
On August 30 2011 03:46 Shockk wrote:This is truly impressive, especially the "microscope" technology involved. Let me quote that part of the article: Show nested quote +An atomic force microscope (AFM) isn't anything like an optical microscope that you simply look through to make small things appear bigger. Instead, it's more like a very very very very very small bit of charcoal that you can rub on tracing paper placed over a surface to view carved patterns that you wouldn't otherwise be able to see. The tip of an AFM (also called a probe) is so small that you need an electron microscope to even see it: To operate, the tip of the AFM moves across a surface, and when it encounters an atom or a molecule, the tip bumps up a little bit as it passes over. This jiggles a laser beam, which records precisely how much the tip was deflected. By making a bunch of passes, the AFM can gradually build up a sort of topographic map of a surface. It's also possible to place a single atom on the very tip of the AFM's probe, and by watching how that atom interacts with the atoms that it passes over, you can tell what's underneath. Just imagine the level of precision neccessary to develop, manufacture, operate and record all of that. And think about the possibilites we could have if such machines couldn't just be used to scan, but instead to modify or create things on that scale.
actually you can write your name in atoms already. at least our prof told us so 
ah damn. just realized someone else mentioned it already.
|
okay so wait....is this like a series of photos compiled over time to create the probability of where the electron is? ie. the cloud. Cuz I thought that if you took a photograph of an electron it can't be actually done since it changes the state of energy that it would be at giving it an inaccurate photo. Heismann's principle or w/e....
So did they somehow overcome this hurdle? That's fucking sick but confuses me.....xplain to me physics majors plzzz
|
I still remember my first electronics class, where in the first lecture this gem of buffoonery was uttered: "Remember, electrons don't just go wherever they want. They travel in well defined orbits."
Down the road, this should lead to some pretty awesome advances in molecular modeling. Exciting times for science!
|
I dont understand. How can they be sure this is an electron. I would think there should be "-" label on it.
On a side note, AFMs are a bitch to use. I broke that tip 3 times during my lab course and in the end I only got a picture of a giant grain of dirt. Thank god I dont have to deal with that anymore, you need so much patience.
|
On August 30 2011 04:42 Zedders wrote:
okay so wait....is this like a series of photos compiled over time to create the probability of where the electron is? ie. the cloud. Cuz I thought that if you took a photograph of an electron it can't be actually done since it changes the state of energy that it would be at giving it an inaccurate photo. Heismann's principle or w/e....
So did they somehow overcome this hurdle? That's fucking sick but confuses me.....xplain to me physics majors plzzz
This is actually the compiled data from the interaction of a single carbon dioxide molecule with this carbon polymer. From the interaction they determined the space where there is NO probability to find an electron therefore the parts left out (white parts) gave us the molecular orbitals. And you're correct, this is not a photo but a representation of their result from the experiment. However, as far as I know the MOs have been only justified by mathematical equations thus making this experiment so important.
|
This is awesome. Science is so cool.
|
I do not know exactly what I am looking at but it is damn awesome nonetheless. Imaging technology today is crazy!
|
This is really mindblowing!
And to show REALLY how far the researchers have gone, here is a somewhat easy comparison:
If the nucleus would be the basketball, electron would be like a needle tip.
To see, how big an atom would be like that, you need to put the needle tip away for about 10 miles from the basketball!
To show how fast the electron circles around the nucleus,
i can't even think of a number that big to show the speed of the needle tip circling around the basketball...
|
most of the time i hate mankind. sometimes - like this time - i love mankind, as well as being part of them.
go humans!! awesome stuff :D
|
Electrons in my mind will always now be linked to 'No Homo For L(o)mo.' Hwaseung Oz forever!
|
Pretty amazing to see firsthand what the math predicted so many years ago. This is awesome.
|
|
|
On August 30 2011 04:03 TangJuice wrote:Show nested quote +On August 30 2011 03:46 Shockk wrote:This is truly impressive, especially the "microscope" technology involved. Let me quote that part of the article: An atomic force microscope (AFM) isn't anything like an optical microscope that you simply look through to make small things appear bigger. Instead, it's more like a very very very very very small bit of charcoal that you can rub on tracing paper placed over a surface to view carved patterns that you wouldn't otherwise be able to see. The tip of an AFM (also called a probe) is so small that you need an electron microscope to even see it: To operate, the tip of the AFM moves across a surface, and when it encounters an atom or a molecule, the tip bumps up a little bit as it passes over. This jiggles a laser beam, which records precisely how much the tip was deflected. By making a bunch of passes, the AFM can gradually build up a sort of topographic map of a surface. It's also possible to place a single atom on the very tip of the AFM's probe, and by watching how that atom interacts with the atoms that it passes over, you can tell what's underneath. Just imagine the level of precision neccessary to develop, manufacture, operate and record all of that. And think about the possibilites we could have if such machines couldn't just be used to scan, but instead to modify or create things on that scale. WOO nano machines!!! GOGO BORG!!!
The dangers of nano-particles are a tricky thing. They can stick to various parts of the biological machine that is you and negatively affect your functionality (make you ill or kill you).
There seems to be consensus that, although one should be aware of materials containing fixed nanoparticles, the immediate concern is with free nanoparticles.
Nanoparticles are very different from their everyday counterparts, so their adverse effects cannot be derived from the known toxicity of the macro-sized material. This poses significant issues for addressing the health and environmental impact of free nanoparticles.
http://en.wikipedia.org/wiki/Impact_of_nanotechnology#Health_and_safety_impact_from_nanoparticles
|
i dnt understand science =(
|
while it is good proof of models, these are not pictures of electrons in orbit, nor do electrons "orbit" the nucleus
|
Wow, this is quite interesting. I'll have to share it with friends. Thanks for the post OP.
|
Lol, my asian scientist dad is saying: "Everybody knows this. These kinds of pictures have been taken before."
XD
Anyways, looks very interesting!
|
lol should I even be surprised at the amount of people laughing about HOMO?
also it is important to note that this only worked in a certain case of molecule, namely one that has a high aspect ratio laterally. think of it like a sheet of paper
|
At first glance I read the title to say "First-Ever Images of an Election in Orbit" I was like WTF!
Awesome news anyway!
|
This is incredible! I love how far science goes every day without us noticing most of it. Humanity can really do awesome things.
|
well we learn this in school already. now there's just proof of it. im not that excited.
|
ok.... i see there is some explaining to do here. just as a bit of a reference, i work as a PhD student for one of the people who helped obtain the results referenced in this http://gizmodo.com/5346964/ibm-takes-first-3d-image-of-atomic-bonds] article, which is also linked in the gizmodo article in the first post and work in the field of molecular microscopy.
There are some misconceptions and presented so far and id like to rectify that a bit and give a very general explanation on what we see and what it means.
The pictures we see are taken by very advanced microcopes, the techniques used are scanning tunneling mocroscopy (STM) and atomic force microscopy (AFM). though called that, they are not microscopes in the "traditional" sense and do not use light and lenses to peek into the very small. instead, both work by scanning a surface with a very narrow metal tip and recording certain surface properties at different points. these measurements can be combined into pictures of the surface, single measurements being the pixels combining the picture. Through the use of piezo crystals and sofisticated dampening of the tip-sample system as a whole, the motion of the tip can be controlled very precisely, on scales smaller the the diameter of an atom. understanding of both techniques requires a basic familiarity with quantum mechanics, but the wikipedia articles on the subject should be enough to explain how exactly they work.
Since we are doing quantum mechanics (QM), one has to be exceedingly careful when using terms such as "position", "velocity" or "path", since they cannot usually be defined in a meaningful way any more. i cant read the full article referenced in the OP at the moment since im not at the university and dont have access to it, but what we see in the pictures are molecular orbitals. what QM can only predict possibilities and other statistical quantities. orbitals are such a quantity, they represent the possibility to find an electron within a certain volume of space. this quantity can be computed numerically for complex systems such as molecules and these days can be measured by STM. due to electronic properties, namely the pauli exclusion principle, only two electrons can fit into one of these orbitals at a time. they are filled up until all the electrons in the molecule are used up. Thus, wer get the highest occupied molecular orbital, the HOMO, and the lowest unoccupied moelcular orbital, or LUMO. these two are usually involved when the molecule exchanges charge with its surroundings, eg during the formation of chemical bonds, which makes them interesting for study.
what can we learn from this? well, by studying the electronic properties of molecules, we can learn something about their behaviour! and since the STM and AFM also allow us to manipulate single atoms and molecules, we can start building things on this scale. our hope is that by understanding molecular interaction on this small scale, we will be able to build circuitry, memory or other useful things from molecular building blocks. a few advances have already been made in this area, as researchers have found molecules that can act as switches or rectifiers or have other potentially useful properties.
ill be happy to try and answer any questions on the topic!
|
On August 30 2011 03:18 0mar wrote:Show nested quote +On August 30 2011 03:15 ilj.psa wrote:
not really understand the images, if its true its amazing though electrons don't follow an orbit, ithey are pretty much at random places inside an orbital That's been known for about 100 years now...
they do follow an orbit, it's just that to OBSERVE it we have to interfere with its orbit so we can never really know.
|
On August 30 2011 05:58 XiGua wrote:
Lol, my asian scientist dad is saying: "Everybody knows this. These kinds of pictures have been taken before."
XD
Anyways, looks very interesting!
true, but for some reason, few people outside the scientific community have taken notice of this. which is a bit of a shame, seeing how it is a very exciting area of research.
|
This is absolutely stunning news. Wow.
Now chemistry teachers over the world can actually say that this IS the way it works, and not just come with theories :D
|
On August 30 2011 06:49 Nycaloth wrote:
The pictures we see are taken by very advanced microcopes, the techniques used are scanning tunneling mocroscopy (STM) and atomic force microscopy (AFM). though called that, they are not microscopes in the "traditional" sense and do not use light and lenses to peek into the very small. instead, both work by scanning a surface with a very narrow metal tip and recording certain surface properties at different points. these measurements can be combined into pictures of the surface, single measurements being the pixels combining the picture. Through the use of piezo crystals and sofisticated dampening of the tip-sample system as a whole, the motion of the tip can be controlled very precisely, on scales smaller the the diameter of an atom. understanding of both techniques requires a basic familiarity with quantum mechanics, but the wikipedia articles on the subject should be enough to explain how exactly they work.
Since we are doing quantum mechanics (QM), one has to be exceedingly careful when using terms such as "position", "velocity" or "path", since they cannot usually be defined in a meaningful way any more. i cant read the full article referenced in the OP at the moment since im not at the university and dont have access to it, but what we see in the pictures are molecular orbitals. what QM can only predict possibilities and other statistical quantities. orbitals are such a quantity, they represent the possibility to find an electron within a certain volume of space. this quantity can be computed numerically for complex systems such as molecules and these days can be measured by STM. due to electronic properties, namely the pauli exclusion principle, only two electrons can fit into one of these orbitals at a time. they are filled up until all the electrons in the molecule are used up. Thus, wer get the highest occupied molecular orbital, the HOMO, and the lowest unoccupied moelcular orbital, or LUMO. these two are usually involved when the molecule exchanges charge with its surroundings, eg during the formation of chemical bonds, which makes them interesting for study.
Thanks for the insight! I don't have much knowledge of this field, but I have some questions about what they're looking at. How are pure "photos" of the HOMO and LUMO (or at least electron probability densities of electrons at these certain energies) obtained? I assume that they excite electrons into the HOMO to get the images of it, but won't there continue to be electrons in lower energy MO's? And wouldn't the same be true for LUMOs? How are they isolating electrons in certain orbitals for viewing while not sensing all of the other electrons in the molecule?
|
Wow, this is an awesome step forward, hopefully more innovations to come soon
|
On August 30 2011 07:06 The_PhaCe wrote:
This is absolutely stunning news. Wow.
Now chemistry teachers over the world can actually say that this IS the way it works, and not just come with theories :D
well, the first thing my prof said was something along the lines "the funny part of physics is that you can't really proof anything. If your experiment shows that it's wrong, than your theory is most likely wrong. However if your experiment got the results you're expecting the only thing you can say is, that maybe (!) you're not wrong but you won't be able to say that you're right" sooo kinda :p
But yeah really stunning.
|
On August 30 2011 06:51 Vei wrote:Show nested quote +On August 30 2011 03:18 0mar wrote:On August 30 2011 03:15 ilj.psa wrote:
not really understand the images, if its true its amazing though electrons don't follow an orbit, ithey are pretty much at random places inside an orbital That's been known for about 100 years now... they do follow an orbit, it's just that to OBSERVE it we have to interfere with its orbit so we can never really know.
Nah. At least not the same sort of orbit a planet has around the sun. Because a circular motion means constant acceleration, and when you accelerate an electron, it emit radiation, meaning it would lose energy at a very rapid rate. I don't remember the exact numbers, but atoms would only last a ridiculously small amount of time if electrons actually moved around an orbit. At least this is the main reason that people started to think about other models than the rutherford-atom model, which basically is electrons moving like planets.
|
On August 30 2011 03:18 0mar wrote:Show nested quote +On August 30 2011 03:15 ilj.psa wrote:
not really understand the images, if its true its amazing though electrons don't follow an orbit, ithey are pretty much at random places inside an orbital That's been known for about 100 years now...
They knew about atoms in 1911?
Source please.
|
When i took orgo i couldn't stop thinking about homo/lumo -> homo for lomo T.T
|
Just to sum up for everyone...
We can never know where electrons exactly are without disturbing their velocity/path whatever. Heisenberg uncertainty principle bla bla bla. Instead we have this "probability function" idea.
Think of a flat plane in the x-y axes, and then for every x and y, give it a specific height. So what you have now resembles a flat landscape with mountains and hills poking up. The height at any point represents the probability that an electron will be there if you randomly check for it.
+ Show Spoiler +
If you're good with mental pictures, you can try and visualize this in 3D with color (for every point x,y,z, associate a color that represents probability, e.g. red = high probability and blue = low probability).
Now think of the AFM as a needle, suspended from the sky, that sweeps through this landscape at a specific height, pretty low. If you hit something, you know that an electron has a pretty good chance of hanging out there fairly regularly. You can trace the borders of these hills and make a "topographical map" of sorts of the probability function.
They took data on this atom with the AFM, filled in the places where they hit something with white, and left everything else dark. What you have left is the white part represents a high probability of finding an electron there, and the black parts mean that it would be exceedingly rare to find an electron there.
What's cool is this happens to match the probability fields that people have been predicting for quite a while. I apologize for some of the gross oversimplifications that physicists will feel I have made, but this interpretation has served me pretty well as an engineer.
|
On August 30 2011 11:37 Ympulse wrote:Show nested quote +On August 30 2011 03:18 0mar wrote:On August 30 2011 03:15 ilj.psa wrote:
not really understand the images, if its true its amazing though electrons don't follow an orbit, ithey are pretty much at random places inside an orbital That's been known for about 100 years now... They knew about atoms in 1911? Source please.
http://en.wikipedia.org/wiki/John_Dalton
They knew about atoms in 1911.
http://en.wikipedia.org/wiki/Ernest_Rutherford
He thought electrons moved in orbits around atoms *about* one hundred years ago.
|
On August 30 2011 11:37 Ympulse wrote:Show nested quote +On August 30 2011 03:18 0mar wrote:On August 30 2011 03:15 ilj.psa wrote:
not really understand the images, if its true its amazing though electrons don't follow an orbit, ithey are pretty much at random places inside an orbital That's been known for about 100 years now... They knew about atoms in 1911? Source please.
John Dalton postulated the existence of atoms in 1805 to explain the Law of Multiple Proportions.
In 1865, Josef Loschmidt measured the sizes of the molecules that make up air.
In 1897, J. J. Thompson discovered the electron.
In 1913, Niels Bohr proposed the Bohr model, which consists of electrons orbiting an atom's nucleus, at fixed distance orbits.
In 1926, Schrödinger proposed that electrons behave as waves, instead of particles, orbiting an atom's nucleus in an orbital cloud, rather then at fixed distance orbits.
So yeah, we've known about atoms for a while. These pictures are important, because they confirm our theories.
|
On August 30 2011 11:37 Ympulse wrote:Show nested quote +On August 30 2011 03:18 0mar wrote:On August 30 2011 03:15 ilj.psa wrote:
not really understand the images, if its true its amazing though electrons don't follow an orbit, ithey are pretty much at random places inside an orbital That's been known for about 100 years now... They knew about atoms in 1911? Source please.
Not exactly this, but for example the Rutherford-Bohr Atom modell is from 1913. The modern Orbitalmodel with electrons as probability areas is from 1926. The general concept of atoms is known since the 19th century. For sources, just go to wikipedia and look at their sourcelist, but you can find this in about every book about atomic models.
|
I was blown away that I can look at that picture. The blurry picture. And count the nodes in the HOMO and LUMO. And see how well it agrees with theory. FINALLY! MO THEORY IS THE ONE THAT ISN"T A BLOODY LIE!
|
Crazy when you're told as a kid that we can't actually see it yet, and then one day we can see it. Just makes it that much cooler.
|
This is really good for Nanotechnology. I didn't think it was possible to be able to see electron orbitals clearly. Whether people know it or not, the era of Nanotech is almost upon us.
|
On August 30 2011 08:13 jgoonld wrote:Show nested quote +On August 30 2011 06:49 Nycaloth wrote:
The pictures we see are taken by very advanced microcopes, the techniques used are scanning tunneling mocroscopy (STM) and atomic force microscopy (AFM). though called that, they are not microscopes in the "traditional" sense and do not use light and lenses to peek into the very small. instead, both work by scanning a surface with a very narrow metal tip and recording certain surface properties at different points. these measurements can be combined into pictures of the surface, single measurements being the pixels combining the picture. Through the use of piezo crystals and sofisticated dampening of the tip-sample system as a whole, the motion of the tip can be controlled very precisely, on scales smaller the the diameter of an atom. understanding of both techniques requires a basic familiarity with quantum mechanics, but the wikipedia articles on the subject should be enough to explain how exactly they work.
Since we are doing quantum mechanics (QM), one has to be exceedingly careful when using terms such as "position", "velocity" or "path", since they cannot usually be defined in a meaningful way any more. i cant read the full article referenced in the OP at the moment since im not at the university and dont have access to it, but what we see in the pictures are molecular orbitals. what QM can only predict possibilities and other statistical quantities. orbitals are such a quantity, they represent the possibility to find an electron within a certain volume of space. this quantity can be computed numerically for complex systems such as molecules and these days can be measured by STM. due to electronic properties, namely the pauli exclusion principle, only two electrons can fit into one of these orbitals at a time. they are filled up until all the electrons in the molecule are used up. Thus, wer get the highest occupied molecular orbital, the HOMO, and the lowest unoccupied moelcular orbital, or LUMO. these two are usually involved when the molecule exchanges charge with its surroundings, eg during the formation of chemical bonds, which makes them interesting for study.
Thanks for the insight! I don't have much knowledge of this field, but I have some questions about what they're looking at. How are pure "photos" of the HOMO and LUMO (or at least electron probability densities of electrons at these certain energies) obtained? I assume that they excite electrons into the HOMO to get the images of it, but won't there continue to be electrons in lower energy MO's? And wouldn't the same be true for LUMOs? How are they isolating electrons in certain orbitals for viewing while not sensing all of the other electrons in the molecule?
To understand how these pictures are made, one has to understand how the machine that takes them works. The scanning tunneling microscope (STM) uses the so called tunneling effect in quantum mechanics. Remember the concept of uncertainty? In QM, we can no longer say where exactly something is with certainty, instead, objects like electrons are "smeared out" over an area of space. These areas will extend beyond the conceptual limits of any given body, say a metal plate or a tip. If we approach plate and tip to one another until these areas of "smeared out" electrons overlap, the electrons can pass from one contact to the other, even though there is no electrical contact between the two. This is quantum tunneling.
By applying an electrical tension between the two contacts, we can give a preferred direction to the tunneling of electrons and measure the resulting tunneling current. This is what an STM does: by scanning a surface and measuring the tunneling current at many points, we can construct a picture of that surface much like a computer screen constructs an image from many pixels.
How does this allow us to image molecular orbitals? if we put molecules on top of the surface that is scanned in the microscope, the picture changes a bit. By varying the electric tension we apply, we can vary the energy of the electrons participating in the tunneling process. If there are MOs in the window of energy we are looking at, the tunneling current will be enhanced because the electrons dont have to pass from tip to sample in one go, but can instead tunnel in two smaller steps, into the molecule first and then from there into the surface. So by scanning the voltage as well, we will observe sharp increases in the tunneling current every time that we pass the energy of a molecular orbital! In the first derivative of the current, these steps are transformed into peaks: they show the position of the MOs in the energetical spectrum.
This is what you see in the pictures: a spatial map of the first derivative of the tunneling current measured between a metal tip and a metal surface on which molecules have been deposited, taken at the energy of the HOMO and LUMO, respectively.
hope that helps!
|
On August 30 2011 19:11 Nycaloth wrote:Show nested quote +On August 30 2011 08:13 jgoonld wrote:On August 30 2011 06:49 Nycaloth wrote:
The pictures we see are taken by very advanced microcopes, the techniques used are scanning tunneling mocroscopy (STM) and atomic force microscopy (AFM). though called that, they are not microscopes in the "traditional" sense and do not use light and lenses to peek into the very small. instead, both work by scanning a surface with a very narrow metal tip and recording certain surface properties at different points. these measurements can be combined into pictures of the surface, single measurements being the pixels combining the picture. Through the use of piezo crystals and sofisticated dampening of the tip-sample system as a whole, the motion of the tip can be controlled very precisely, on scales smaller the the diameter of an atom. understanding of both techniques requires a basic familiarity with quantum mechanics, but the wikipedia articles on the subject should be enough to explain how exactly they work.
Since we are doing quantum mechanics (QM), one has to be exceedingly careful when using terms such as "position", "velocity" or "path", since they cannot usually be defined in a meaningful way any more. i cant read the full article referenced in the OP at the moment since im not at the university and dont have access to it, but what we see in the pictures are molecular orbitals. what QM can only predict possibilities and other statistical quantities. orbitals are such a quantity, they represent the possibility to find an electron within a certain volume of space. this quantity can be computed numerically for complex systems such as molecules and these days can be measured by STM. due to electronic properties, namely the pauli exclusion principle, only two electrons can fit into one of these orbitals at a time. they are filled up until all the electrons in the molecule are used up. Thus, wer get the highest occupied molecular orbital, the HOMO, and the lowest unoccupied moelcular orbital, or LUMO. these two are usually involved when the molecule exchanges charge with its surroundings, eg during the formation of chemical bonds, which makes them interesting for study.
Thanks for the insight! I don't have much knowledge of this field, but I have some questions about what they're looking at. How are pure "photos" of the HOMO and LUMO (or at least electron probability densities of electrons at these certain energies) obtained? I assume that they excite electrons into the HOMO to get the images of it, but won't there continue to be electrons in lower energy MO's? And wouldn't the same be true for LUMOs? How are they isolating electrons in certain orbitals for viewing while not sensing all of the other electrons in the molecule? To understand how these pictures are made, one has to understand how the machine that takes them works. The scanning tunneling microscope (STM) uses the so called tunneling effect in quantum mechanics. Remember the concept of uncertainty? In QM, we can no longer say where exactly something is with certainty, instead, objects like electrons are "smeared out" over an area of space. These areas will extend beyond the conceptual limits of any given body, say a metal plate or a tip. If we approach plate and tip to one another until these areas of "smeared out" electrons overlap, the electrons can pass from one contact to the other, even though there is no electrical contact between the two. This is quantum tunneling. By applying an electrical tension between the two contacts, we can give a preferred direction to the tunneling of electrons and measure the resulting tunneling current. This is what an STM does: by scanning a surface and measuring the tunneling current at many points, we can construct a picture of that surface much like a computer screen constructs an image from many pixels. How does this allow us to image molecular orbitals? if we put molecules on top of the surface that is scanned in the microscope, the picture changes a bit. By varying the electric tension we apply, we can vary the energy of the electrons participating in the tunneling process. If there are MOs in the window of energy we are looking at, the tunneling current will be enhanced because the electrons dont have to pass from tip to sample in one go, but can instead tunnel in two smaller steps, into the molecule first and then from there into the surface. So by scanning the voltage as well, we will observe sharp increases in the tunneling current every time that we pass the energy of a molecular orbital! In the first derivative of the current, these steps are transformed into peaks: they show the position of the MOs in the energetical spectrum. This is what you see in the pictures: a spatial map of the first derivative of the tunneling current measured between a metal tip and a metal surface on which molecules have been deposited, taken at the energy of the HOMO and LUMO, respectively. hope that helps!
Thanks for the insight 
|
Feel pretty geeky knowing all that stuff (Chemical Engineering). Just seeing the AFM tip taken out and fitted in, and seeing what kind of images it can get of my sample.
Quantum mechanics powa!
To understand how these pictures are made, one has to understand how the machine that takes them works. The scanning tunneling microscope (STM) uses the so called tunneling effect in quantum mechanics. Remember the concept of uncertainty? In QM, we can no longer say where exactly something is with certainty, instead, objects like electrons are "smeared out" over an area of space. These areas will extend beyond the conceptual limits of any given body, say a metal plate or a tip. If we approach plate and tip to one another until these areas of "smeared out" electrons overlap, the electrons can pass from one contact to the other, even though there is no electrical contact between the two. This is quantum tunneling.
By applying an electrical tension between the two contacts, we can give a preferred direction to the tunneling of electrons and measure the resulting tunneling current. This is what an STM does: by scanning a surface and measuring the tunneling current at many points, we can construct a picture of that surface much like a computer screen constructs an image from many pixels.
How does this allow us to image molecular orbitals? if we put molecules on top of the surface that is scanned in the microscope, the picture changes a bit. By varying the electric tension we apply, we can vary the energy of the electrons participating in the tunneling process. If there are MOs in the window of energy we are looking at, the tunneling current will be enhanced because the electrons dont have to pass from tip to sample in one go, but can instead tunnel in two smaller steps, into the molecule first and then from there into the surface. So by scanning the voltage as well, we will observe sharp increases in the tunneling current every time that we pass the energy of a molecular orbital! In the first derivative of the current, these steps are transformed into peaks: they show the position of the MOs in the energetical spectrum.
This is what you see in the pictures: a spatial map of the first derivative of the tunneling current measured between a metal tip and a metal surface on which molecules have been deposited, taken at the energy of the HOMO and LUMO, respectively.
hope that helps!
You have a gift for explaining things haha. I know STM and STILL couldn't convey what it does for anybody below college chemistry! Good job
|
On August 30 2011 12:21 DtorR wrote:
This is really good for Nanotechnology. I didn't think it was possible to be able to see electron orbitals clearly. Whether people know it or not, the era of Nanotech is almost upon us.
Nanotech has been with us for many years. The linewidth used to write transistors for processors is sub 100nm these days. Bit sizes on modern harddrives is sub 100nm as well. These pictures are interesting on their own, but I'm skeptical on the revolutionizing aspect.
|
On August 30 2011 20:11 OrchidThief wrote:Show nested quote +On August 30 2011 12:21 DtorR wrote:
This is really good for Nanotechnology. I didn't think it was possible to be able to see electron orbitals clearly. Whether people know it or not, the era of Nanotech is almost upon us. Nanotech has been with us for many years. The linewidth used to write transistors for processors is sub 100nm these days. Bit sizes on modern harddrives is sub 100nm as well. These pictures are interesting on their own, but I'm skeptical on the revolutionizing aspect.
Single molecules are still a good deal smaller then that, on the scale of a few nm, one to two orders of magnitude lower then the bit sizes you mentioned. But miniaturisation is only one aspect of this research, maybe the most obvious. if we can build resistors, wires and capacitors from such small building blocks, electronics would get a good deal smaller yet again.
But the real prospect is efficient use of resources. Over the past few years, you may have read news articles about material shortage in the high tech industry. construction of displays, memories and chips often requires rather exotic raw materials which are rare and hard to get to and the prices have been rising steadily for a long time. If we can find new ways to do the same thing with easier to obtain materials, thats a good thing. most of the molecules looked at in current research are composed of mostly carbon, oxygen, hydrogen and nitrogen with metal centers. Thats one atom of metal per molecule.
The chemists have a unit to measure the quantity of a substance: the mole. A mole is roughly six times ten to the twentythird power, or a huge bloody lot. This is the scale on which molecules can be produced with ease with very little effort. This is the second big perspective: if we understand the dynamics of single molecules to a degree where we can build things from them, our production capabilities would be infinite for all practical purposes, while using less raw materials, less energy and probably less time then they do now.
|
Nycaloth, I just want to thank you for your explanations. I only did Physics in high school but I feel smarter just by reading your explanations. This is pretty cool.
|
Homo..........wat. ) )
|
On August 30 2011 20:50 Nycaloth wrote:Show nested quote +On August 30 2011 20:11 OrchidThief wrote:On August 30 2011 12:21 DtorR wrote:
This is really good for Nanotechnology. I didn't think it was possible to be able to see electron orbitals clearly. Whether people know it or not, the era of Nanotech is almost upon us. Nanotech has been with us for many years. The linewidth used to write transistors for processors is sub 100nm these days. Bit sizes on modern harddrives is sub 100nm as well. These pictures are interesting on their own, but I'm skeptical on the revolutionizing aspect. Single molecules are still a good deal smaller then that, on the scale of a few nm, one to two orders of magnitude lower then the bit sizes you mentioned. But miniaturisation is only one aspect of this research, maybe the most obvious. if we can build resistors, wires and capacitors from such small building blocks, electronics would get a good deal smaller yet again. But the real prospect is efficient use of resources. Over the past few years, you may have read news articles about material shortage in the high tech industry. construction of displays, memories and chips often requires rather exotic raw materials which are rare and hard to get to and the prices have been rising steadily for a long time. If we can find new ways to do the same thing with easier to obtain materials, thats a good thing. most of the molecules looked at in current research are composed of mostly carbon, oxygen, hydrogen and nitrogen with metal centers. Thats one atom of metal per molecule. The chemists have a unit to measure the quantity of a substance: the mole. A mole is roughly six times ten to the twentythird power, or a huge bloody lot. This is the scale on which molecules can be produced with ease with very little effort. This is the second big perspective: if we understand the dynamics of single molecules to a degree where we can build things from them, our production capabilities would be infinite for all practical purposes, while using less raw materials, less energy and probably less time then they do now.
Yes. I realize single molecules are several order of magnitude smaller than the examples I used, my point was, nanotech has been alive and kicking for several decades. Spintronics and single electron transistors have large potentials, but however amazing these images are, they're not just going to completely revolutionize -- anything, because all engineering today is a complex multidisciplinary process where verification on the atomic level is a minor part.
|
|
|
|
|
|
![[image loading]](http://i.imgur.com/5vixq.jpg)
![[image loading]](http://dvice.com/assets_c/2011/08/atom_force_micro-thumb-330x264-69456.jpg)
![[image loading]](http://metamodern.com/b/wp-content/uploads/2009/03/atom_interchange_anim.gif)